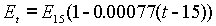
At the dawn of the electrical age it was quickly realized that a reproducible standard of voltage was needed. Ideally, that standard would be insensitive to temperature changes, current flow, and aging. It would have a long life. It should also be possible for workers in widely separated labs to construct the standard with a high degree of reproducibility. A voltaic cell was the obvious choice of the time, however construction of a cell meeting those requirements was to become the subject of much research.
One of the many early cells used for a voltage standard was the Daniell Cell (1836). A Daniell Cell consists of a zinc plate immersed in a zinc sulfate solution, and a copper plate immersed in a copper sulfate solution. The two solutions are kept separated by a porous earthen cup, or by gravity, but are in a common vessel. The gravity, or "crowfoot" type of cell was used almost exclusively in telegraph work. Either style produced about 1.08 volts. Though the Daniell cell was used briefly as a voltage standard, it suffered from short life and a non-constant e.m.f. In fact, the Daniell Cell requires that the electrodes be removed from the electrolyte when the cell is not in use.
In 1872 Latimer Clark invented the Clark Cell. It consisted of mercury and zinc amalgam electrodes in a saturated solution of zinc sulfate. This was a big improvement over the Daniell Cell, but suffered from two major imperfections. It had a large temperature coefficient of -0.00115 V/°C, and suffered from cracking where the platinum connections entered the glass envelope. This was caused by the platinum alloying with the zinc amalgam. In spite of its problems, it was reproducible, and became the first commercially successful standard cell.
Lord Rayleigh investigated a form of Clark Cell in 1885 that became known as the Board of Trade Cell of 1894. He established the e.m.f. at any temperature to be:
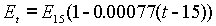
He gave the voltage as 1.434 volts at 15°C, but subsequent investigation has shown this value to be too high by nearly 0.1%. An improved form of the cell was developed at the Reichsanstalt, by Kahle. The correct e.m.f. is 1.4328 international volts, according to Laws Electrical Measurements (1917).
Edward Weston (1850-1936) described the Weston Cell in a patent application in 1891. By substituting cadmium for zinc, and cadmium sulfate for the zinc sulfate electrolyte, he created a cell that largely overcame the deficiencies of the Clark Cell. It was highly reproducible, had a lower temperature coefficient, and didn't crack unless dropped!
The Weston Standard Cell is invariably built in a glass "H" tube. At the bottom of each "H", platinum wires are sealed into the glass to make contact with the electrodes of the cell proper. Packed around one wire is liquid mercury, topped with a paste of mercurous sulfate. This forms the positive electrode. Packed around the other wire is a cadmium/mercury amalgam. This forms the negative electrode. The saturated cells would have a layer of cadmium sulfate crystals above the electrodes, and the un-saturated cells would use various packings to retain the electrodes. The top of the cell is then filled with cadmium sulfate solution.
The purity of chemicals used is critical. The following is from Harris:
"Materials of the highest purity are essential to the construction of satisfactory standard cells. The basic materials- mercury, cadmium, sulfuric acid, and water- are all purified by distillation, starting with the purest materials available. Cadmium sulfate is preferably prepared from cadmium metal and acid, the cadmium being dissolved in nitric acid, crystallized several times as a nitrate, precipitated as cadmium sulfate with redistilled sulfuric acid, and purified by repeated recrystallization as the sulfate. Mercurous sulfate is prepared electrolytically in a darkened room. The cadmium amalgam (10% cadmium) is prepared either electrolyticly or by heating the two metals together."
The finished cell must be mounted in a suitable enclosure to protect it, and to provide measurement terminals. Bakelite cases thermally lagged with copper (aluminum in later cells) are often used, or fully temperature controlled enclosures for sets of saturated cells.
Two forms of the Weston Standard Cell are available: saturated and un-saturated. This refers to the concentration of cadmium sulfate in the electrolyte. The saturated type of cell is the most permanent, however it also has a larger temperature coefficient, and is not portable. It is typically used only for very accurate work, and must be temperature controlled. It is this type of cell that was used by the national laboratories to standardize the value of the volt.
The un-saturated type of cell is more common, as it is portable. The electrode material and mercurous sulfate are held firmly in place with porous plugs, so the cell won't be damaged if inverted. The temperature coefficient can be ignored in most applications, but is given by:
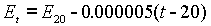
Further variations of both the saturated and un-saturated cell are available in the form of "neutral" or "acid " cells. Acid cells are similar to the neutral of normal cells, with the addition of a small amount of sulfuric acid. The e.m.f. of acid cells is lower than that of normal cells, depending on the concentration of acid. The following formula gives the difference in e.m.f., where N is the normality of the sulfuric acid:
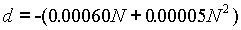
The London Conference on Electrical Units and Standards adopted the Weston Standard Cell as the international standard of voltage in 1908. The National Bureau of Standards, now NIST, followed suite on January 1, 1911.
At that time, the voltage was stated to be 1.01830 International Volts at 20°C. At any other temperature the voltage was calculated using the following formula:
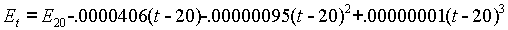
The original work was done by Wolff and published by the NBS in 1908. The coefficients are still considered accurate today.
As can be seen, the cell must be kept at a constant temperature to insure accurate results. If that requirement is met, and if the cell was constructed with sufficiently pure materials, the voltage will remain stable to better than 1 ppm for long periods of time. Some of the cells maintained by the NIST are 70-80 years old, and are still stable and reliable, though no longer officially used as a voltage reference.
By today's standards of solid state ruggedness and reliability, standard cells are finicky, delicate, and prone to damage through misuse. The simple application of a voltmeter to measure the cell will draw enough current to change the value of the cell, and will do permanent damage if kept up for any length of time. Modern high impedance DVMs lessen the danger, but standard cells should never be measured or used in this manner. (This statement obviously does not include solid state scanners meant for the purpose, or "electrometer input" DVMs having input impedances of hundreds of megohms.)
Any current flow into or out of the cell is detrimental. One µA for several minutes can produce measurable changes in e.m.f. Under no circumstances should more than 100 µA ever be drawn. The following table shows the result of various currents and the associated recovery time:
| current | time in minutes | e.m.f. loss at → | time |
| 20 µA | 3 | 10 µV | 1 hr. |
| 100 µA | 3 | (unspecified) | 24 hrs. |
| short circuit | 30 | 1000 µV | 24 hrs. |
| short circuit | 30 | 75 µV | 5 weeks |
Standard cell measurements are almost always made in the "null" configuration. Two cells are connected "back to back ", and the voltage difference between the two is measured. The measurement is always as brief as possible.
It takes a minimum of three cells to maintain the volt with any degree of reliability. With one cell, there is no way to know if a change has occurred. With two cells, a change in either one will be detected, but there is no way to determine which one. With three cells, the errant cell can be identified. In addition, one cell can be sent off for certification, then compared on its return to be sure no shift or damage has occurred. The following rules are condensed from Harris' Electrical Measurements (1952):
Much has been published concerning the life of un-saturated cells, but information of the saturated cells is less common. This is likely because, with proper care, they can last for decades.
The un-saturated cell should have an e.m.f. of 1.0190 volts @ 20°C when new. When the voltage drops to 1.0183 volts, the cell should be discarded, as it is no longer stable. Most reputable labs will not certify a cell below this value.
The NBS recorded data on approximately 600 un-saturated cells. About 5% showed an increase of e.m.f. with time, the average change being 28 µV/year. The remaining 95% decrease in e.m.f., at an average of 85 µV/year. Of that group, nearly half changed by more than 50 µV/year, and one fourth by more than 100 µV/year. That data suggests that certification at yearly intervals is required to insure .01% accuracy. The expected life of un-saturated cells is 7-14 years.
The internal resistance of an un-saturated cell is typically between 80 and 500 ohms, depending on construction. High internal resistance may sometimes develop due to a trapped bubble which can sometimes be freed by gentle tilting and tapping. It is possible to measure the internal resistance, but the procedure should be carried out as rapidly as possible to avoid damage to the cell. If the exact internal resistance isn't needed, the procedure should be avoided.
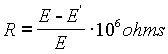
The following BASIC program will calculate standard cell voltages at different temperatures. It is written with generic statements and will need modification to run under any specific implimentation of BASIC. The comments concern a specific cell oven and need not be entered:
'standard cell calculator
INPUT"Enter standard cell voltage at 28 degrees C.";E28
INPUT"Enter new temperature";T
EX=-53.9*(T-28)-0.71*(T-28)^2+0.01*(T-28)^3
PRINT"delta V = ";EX;" microvolts"
EX=EX*1E-6
PRINT"Cell voltage will be ";EX+E28;" volts at ";T;" degrees C."
'comments:
'The formula used above is from Eppley's literature. They adapted
'it for 28 degrees C, as that is the standard cell oven temperature.
'JRL cell ovens run at 37 degrees so hot days aren't a problem. They
'used Eppley cells that are specified at 1.018220 V emf at 20 degrees.
'Applying the formula, the normal output of a JRL cell oven and cells
'is 1.017685 V emf. (new tolerance is about plus or minus 20 uV)
'My JRL cell oven and cells read about 1.017725.
'the cell coefficient between 36 and 37 degrees C is 63.8 uV/degree
'the cell coefficient between 37 and 38 degrees C is 64.68 uV/degree
'the international formula using 20 degrees as a reference uses
'numerical coefficients of .0000406, .00000095, and .00000001
There are few Weston Standard Cells in use today, and for good reason. Most applications are easily met with simple solid state references. ICs having initial accuracy of .01% and temperature coefficients of one or two parts per million are easily obtained. Ovenized zener diode references do even better, though at substantially higher cost. The only performance advantage a standard cell has today is low noise, and even that isn't a serious issue.
An even larger reason for the decline of standard cells is the environmental concern over the mercury and cadmium they contain. These are both toxic heavy metals, and must be treated as hazardous waste, come disposal time. The manufacture of mercury-containing batteries has been outlawed almost everywhere, and standard cells fall under this ruling. Eppley, one of the best known makers of standard cells ceased production many years ago. One can assume that almost all un-saturated cells are no longer usable.
The final nail in the standard cell coffin was the development of the Josephson Junction Array at NIST. This is a super-conducting semiconductor array that generates voltage steps in response to microwave radiation of known frequency. It is a primary standard and multiple units have shown agreement to parts per billion.
Eppley, catalog/data sheet (1993)
Julie Research, SCO-3 instruction manual (1966)
NIST, conversations with Norman Belecki, Electricity Division (1995-6)
Stout, Basic Electrical Measurements (Prentice-Hall, 1950)
Smith, Electrical Measurements in Theory and Application (McGraw-Hill, 1948)
Laws, Electrical Measurements (McGraw-Hill, 1917)
Michels, Advanced Electrical Measurements (D. Van Nostrand, 1941)
Harris, Electrical Measurements (Wiley, 1952)
Timbie & Bush, Electrical Engineering
Dawes, Electrical Engineering, Vol. 1 (McGraw-Hill, 1937)
Hawkens Electrical Guide #2